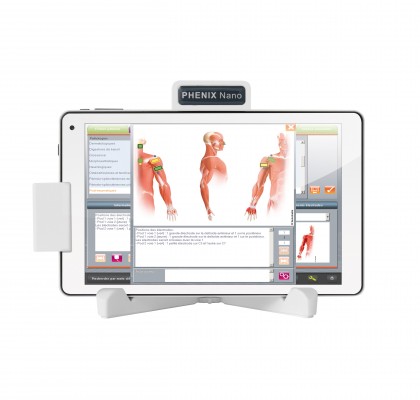
About product : PHENIX NANO Portable PHYSIO URO
Delivery
Types of associated treatments
See aswell
Detailed description
Biofeedback has been a long time reserved for pelvic floor muscle training while you are over 80% to measure the interest in the rehabilitation of the musculoskeletal system.
The biofeedback allows the patient to become aware of an abnormal physiological condition and guide in its correction. It also allows the therapist to objectify the evolution and the acquisition of the correction.
Whether for amyotrophies, muscle considerations, the locking deficits, contractures and spasticity, the effectiveness of electrostimulation EMG-biofeedback torque will surprise you. With just two electrodes, stimulation creates sensory experience and biofeedback can reproduce in retro control. Your patients become actors of their treatment and their motivation increases.
With PHENIX NANO Physio Uro, we make everyone therapists combination of these two techniques in a portable and transportable device. The richness screens biofeedback and stimulation currents, this combination can significantly accelerate the processes of neuromuscular reprogramming.
With PHENIX NANO Physio Uro, the practice of biofeedback is simple and optimizes your results in all the pathologies that require improvement or a reprogramming of the motor command. Its handling patient guide in a static or dynamic work and the patient can be placed in functional situations.
Using the PHENIX NANO Physio Uro
The PHENIX NANO Physio Uro is both easy to use and very rich. With its selection tools it suits your needs.
To save even more time, you can also sort the programs will bring up the electrostimulation programs ...
...or Biofeedback
...or those who associate electrostimulation and biofeedback
Comfort and efficiency of electrostimulation
By removing the latency between the negative pulse and the positive pulse, the repolarization of the cell is immediate, the stimulation becomes more comfortable, allowing muscle recruitment ever.
DISEASES
AMYOTROPHIC - CIRCULATORY AND TROPHIC - CONTRACTURES MUSCLE -DERMATOLOGIQUES - DIGESTIVE TRANSIT - MORPHOESTHETIQUES - -OSTEOARTICULAIRES NEUROLOGIC AND TENDON - POST-TRAUMATIC (ankle sprain, knee sprain, broken wrist sprain, dislocation of the elbow, NCB) - RHEUMATOLOGICAL (algoneurodystrophy , capsulitis, positional Neck pain, patellar chondral, Coxarthrose, DUPUYTREN, Gonarthrosis, Lombo-sciatica, HSP) - SPORT
FEMALE PERINEUM : urinary incontinence in effort-bladder instability - the urge incontinence - the dyssynergies - postpartum disorders - anorectal disorders - incontinence gases - rehabilitation reflexes of anal continence - the episiotomy pain - pain dyspareunia - vaginismus - the muscle and tendon pain intraoperative and postpartum - preparation for surgery urethrovesical
PERINEUM MEN : after prostatectomy incontinence - the urge incontinence with urethral or bladder instability - preparation for prostate surgery - disErection - premature ejaculation - reflexes rehabilitation anal continence
Therapeutic Assistant :
Simply and only with one clinical sign, the wizard will help you find in a few seconds an analgesic program exito engine, draining, etc.
You observe, you question, you are evaluating the clinical signs :
- Urogynecology :
SUI, urgency, leaking, pain ...
- Physiotherapy :
Pain, inflammation, atrophy, edema ...
The wizard finds and organizes the answers in a comprehensive and coherent treatment.
The PHENIX NANO Physio is delivered with :
- 1 POD Stim/Bio
- 1 output cable with 4 wires
- 1 tablet support
- 1 Lithium Polymer Rechargeable Extended *
- 1 power supply medical standards
- 1 user manual
Options :
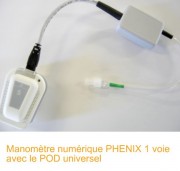
The PHENIX manometric 1 channel and POD Universal : In many therapeutic situations (anal sphincter deficiencies, constipation, rectal dissynergies duplex ...) it is necessary to use an inflatable probe (vaginal or anal) connected to a manometric module. PHENIX digital manomectric displays on screen the pressure recorded at the recording Biofeedback balloon and practice exercises.
Standards :
CE Marking 0459
Class IIb device designed and manufactured according to European directive 93/42 / EC